
StudySmarter - The all-in-one study app.
4.8 • +11k Ratings
More than 3 Million Downloads
Free
Americas
Europe



Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persönlichen Lernstatistiken
Jetzt kostenlos anmeldenTake an atom. Prise it open and look inside. Of course, you can’t really cut open an atom like that - they are far too small to be seen, apart from under the most powerful electron microscopes. But if you could, what would you see?
In the centre of the atom, you find a dense mass called the nucleus. Imagine 6 million cars squashed up together into a lump just 1 foot wide, 1 foot deep and 1 foot tall. That’s how dense the nucleus is. It contains protons, which are positively charged, and neutrons, which have a neutral charge.
Surrounding the nucleus, you find tiny negatively charged particles called electrons. Scientists like to imagine them as particles, but in reality they behave like waves at times - almost like a light wave. We imagine them whizzing around the nucleus in circular paths called orbitals. But although this sounds busy, the atom is mostly empty space. If an atom was the size of a cathedral, how big do you think its nucleus would be? The answer - the size of a fly.
 The structure of an atom[1] with electrons marked in red, protons in blue and neutrons in green.Plazmi, CC BY-SA 4.0, via Wikimedia Commons
The structure of an atom[1] with electrons marked in red, protons in blue and neutrons in green.Plazmi, CC BY-SA 4.0, via Wikimedia Commons
The behaviour of atoms depends on electrons. All reactions involve moving electrons about. Sometimes this is from one atom to another. In other cases, this is from an atom to a delocalised system, where the electrons don’t belong to any one specific atom. The more easily an atom gains or loses electrons, the more likely it is to react with another substance. Atoms can react together to form a variety of different molecules and structures, ranging from simple diatomic molecules to vast lattices.

Right-a diatomic molecule, Left- a giant lattice, Christinelmiller, CC-BY-SA 4.0, Wikimedia Commons
This brings up questions: why do atoms lose or gain electrons? Why don’t they move their protons about? What do lattices form and what forces hold them together? Why do some substances react so quickly and yet others are practically inert, meaning they don’t react at all?
To answer these questions, we need to take a look at something known as physical chemistry.
Physical chemistry is a branch of chemistry that investigates how substances behave on an atomic or molecular level.
In subjects like biology, you look at how whole organisms work - from their tissues and their organs to how they interact with their environment. In physics, you study topics such as matter, forces and energy. Physical chemistry combines the two. It provides clear explanations as to how fundamental physical laws governing our world cause atoms and molecules to behave, and in turn react to build these organic structures like the heart or brain. It is the stepping stone between simple forces and complex life.
We’ve mentioned atoms above, but what actually are they?
Atoms are the smallest unit of ordinary matter that form a chemical element.
in fact, atoms are the fundamental building blocks of all parts of chemistry, but there are some other pretty important terms you should know about:
In physical chemistry, you’ll study a variety of topics. These range from atomic structure, where you’ll find out more about the particles inside an atom, to kinetics, where you’ll discover how reactions occur and how we can manipulate them. Other topics include acids and bases, amount of substance and equilibria.
You should already know what an atom is. It is the smallest unit of an element that can’t be broken down further by any sort of chemical reaction. But that doesn’t mean that atoms don’t contain their own constituent parts. We explored above how atoms consist of subatomic particles known as protons, neutrons and electrons. In “Atomic Structure”, you’ll learn how these particles are arranged inside atoms. You’ll explore how changing their numbers changes the atom, and you’ll learn to define words such as ion, isotope and ionisation energy.
An ion is an atom that has lost or gained an electron to form a charged particle.
Isotopes are atoms of the same element with different numbers of neutrons.
For example, what is the difference between a hydrogen atom and a helium atom? How can we tell them apart? Can we change from one to the other - and if not, why not?
 Left: a hydrogen atom. Right: a helium atom. Can you spot the differences?commons.wikimedia.org
Left: a hydrogen atom. Right: a helium atom. Can you spot the differences?commons.wikimedia.org
Once you’ve learnt about atoms and elements, you can begin to look at how they react together. But before you do that, you need to understand the basics of reactions and how chemists work with them. In “Amount of Substance”, you’ll cover topics such as molecular mass, empirical formulae and the mole.
Molecular mass is the sum of the atomic masses of all the atoms in a molecule.
Moles have nothing to do with the animal - instead, a mole is a specific quantity of atoms or molecules. They make reaction equations much easier to work with.
 There a 602 sextillion molecules in a mole of any substance. This is known as Avogadro's constant.StudySmarter Originals
There a 602 sextillion molecules in a mole of any substance. This is known as Avogadro's constant.StudySmarter Originals
For example, if we know values such as the mass of a sample and its chemical formula, we can work out not only the number of moles it contains, but how many moles of a product we expect a reaction to produce.
Perhaps you didn’t get as much of your product as expected. You could say that your reaction has a low percentage yield. In “Amount of substance”, you’ll also learn how to calculate this percentage yield and why it is sometimes so low.
We know that atoms react by moving their electrons about. Atoms want to be in the most stable state possible, and they do this by losing or gaining electrons. Too many electrons? They’ll give some up. Not enough? They’ll try to gain a few.
In “Bonding”, you’ll explore some of the ways that atoms shunt their electrons around, from donating them to other atoms to sharing them amongst themselves. Moving electrons forms bonds, and you’ll learn about the different types of bonds between atoms. You’ll also define something called electronegativity.
Electronegativity is an atom’s ability to attract a bonded pair of electrons.
This topic builds on your knowledge of atomic structure. It also incorporates knowledge about forces and attraction. For example, what forces hold a bond together? Why are some bonds much stronger than others?
After looking at bonds from a subatomic point of view, you’ll then consider them on a molecular level. You’ll again apply your knowledge of forces to explain why different molecules have different shapes, and consider how bonding between molecules gives substances such different properties.

Some examples of molecule shapes, Public domain
commons.wikimedia.org
What determines if two substances react together? We call this the feasibility of a reaction, and it is all to do with energy changes. In “Energetics” and “Thermodynamics”, you’ll learn about two related ideas known as enthalpy and entropy.
Enthalpy change is the heat change of a chemical reaction under constant temperature and pressure. Enthalpy is really just a measure of energy.
You’ll explore why some substances give out heat when they react and how we measure this heat in a process called calorimetry. After that, you’ll practise working out energy changes of reactions by drawing enthalpy diagrams. And once you finish this topic, you’ll be able to predict the feasibility of any reaction just by looking at a few simple enthalpy and entropy values.
 An enthalpy diagram for an exothermic reaction[3], Brazosport College, CC BY-SA 3.0, via Wikimedia Commons
An enthalpy diagram for an exothermic reaction[3], Brazosport College, CC BY-SA 3.0, via Wikimedia Commons
Now that we know why atoms react from a chemical point of view, we can turn our attention back to physics. “Kinetics” is the study of the motion of particles and how this affects changing systems. Take iron and water, for example. They react to produce iron(III) oxide. At room temperature, this reaction is very slow. But if you instead react the iron with steam, the reaction happens much faster. Why is this the case?
In “Kinetics”, you’ll learn about the rates of different reactions and how we can manipulate them. Heat is one way of increasing the speed of a reaction, but you’ll also explore other factors such as surface area and concentration. You’ll expand this knowledge in “Rate Equations”. By the end of the topic, you’ll know how to work out the rate of reactions both experimentally and theoretically.
In “Thermodynamics”, you learnt about the feasibility of a reaction. In fact, some reactions are feasible in both directions - there is a forward reaction and a backward reaction.
Let’s look at the white solid ammonium chloride, \( NH_4Cl\) . It breaks down into ammonia gas and hydrochloric acid.
$$ NH_4Cl_{(s)}\rightleftharpoons NH_{3(g)}+ HCl_{(g)} $$
If you leave the reaction alone in a sealed system, there will always be some white solid left, no matter how long you leave it for. The reaction doesn’t go to completion. We say that this type of reaction is reversible - as some of the ammonium chloride breaks down, some is reformed from ammonia and hydrochloric acid. It forms an equilibrium.
A chemical equilibrium is a reaction system with constant concentrations of products and reactants, where the rate of the forward reaction is the same as the rate of the backward reaction.
But what if we don’t want any ammonium chloride? How can we influence the reaction to maximise our yield of ammonia and hydrochloric acid? In “Equilibria”, you’ll learn how changing reaction conditions shifts the reaction to one side or the other. You’ll meet Le Châtelier and find out more about his principle then apply it to industrial examples, such as the production of ethanol. We’ll also explore the equilibrium constants, Kp and Kc. You'll be able to use them to work out the composition of an equilibrium mixture.
Above, we mentioned how iron(III) oxide is formed from iron and water. But what do the roman numerals shown after the iron mean?
Well, they describe something called oxidation states. You’ll learn about these in “Redox”. Oxidation states show how many electrons an atom has lost or gained in a reaction. In oxidation reactions, atoms lose electrons whereas in reduction reactions, atoms gain electrons. We’ll return to the movement of electrons and practice writing half equations to represent this.
You’ll then explore electrochemical cells. Imagine hooking two different metals up to a wire and dipping them in a salt solution. What do you expect to happen? Why is a current produced and how do we predict which direction it will flow? We can use our knowledge of atomic structure and electronegativity to answer these questions.
 An electrochemical cell.StudySmarter Originals
An electrochemical cell.StudySmarter Originals
For the final topic “Acids and bases”, instead of focusing on electron movement, we’ll look at proton movement. You’ll have learnt in “Atomic Structure” that the hydrogen ion, \(H^+\), contains just one proton and no electrons or neutrons, and so we call it a proton. When substances called acids and bases react in reactions known as neutralisation reactions, they move protons around. Neutralisation reactions are extremely common in everyday life - for example, toothpastes neutralise acidic compounds produced by bacteria in our teeth, and baking powder contains a mixture of acids and bases, that when dissolved in solution, produce gases that make cakes rise.
You’ll learn about the pH scale, invented by a Danish biochemist working for a brewing company. You will also find out how to work out the pH of substances before and after neutralisation. Finally, you’ll explore how pH changes in titrations.
 The pH scale. Acidic substances have a low pH and alkaline substances have a high pH.StudySmarter Originals
The pH scale. Acidic substances have a low pH and alkaline substances have a high pH.StudySmarter Originals
Physical chemistry is a branch of chemistry that investigates how substances react on a chemical and molecular level. It deals with topics such as atomic structure, bonding and rate of reaction.
Organic chemistry is a branch of chemistry that studies the structure, properties and reactivity of organic compounds, which are molecules that contain carbon.
Inorganic chemistry is a branch of chemistry that deals with inorganic compounds, which are molecules that aren’t based on carbon.
We can use physical chemistry for many different things, such as predicting and changing the rate of a chemical reaction, predicting molecular shape, and optimising industrial reactions through atom economy and equilibria.
Flashcards in Physical Chemistry3690
Start learningWhich statement is correct?
Protons have a relative mass of 1
Complete the following sentence: electrons are found ___________.
In shells orbiting the nucleus.
What does mass number represent?
The total number of protons and neutrons in an atom.
What does atomic number represent?
The number of protons in an atom.
Which particle determines the chemical properties of an atom?
Electron
Which particle determines the element an atom belongs to?
Proton
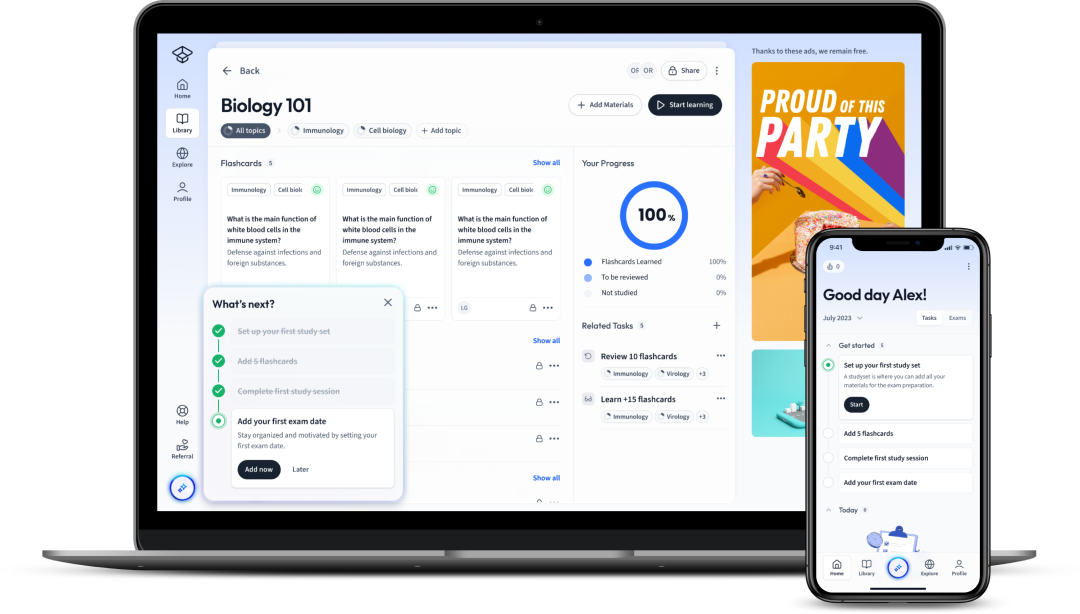
Already have an account? Log in
Open in AppThe first learning app that truly has everything you need to ace your exams in one place
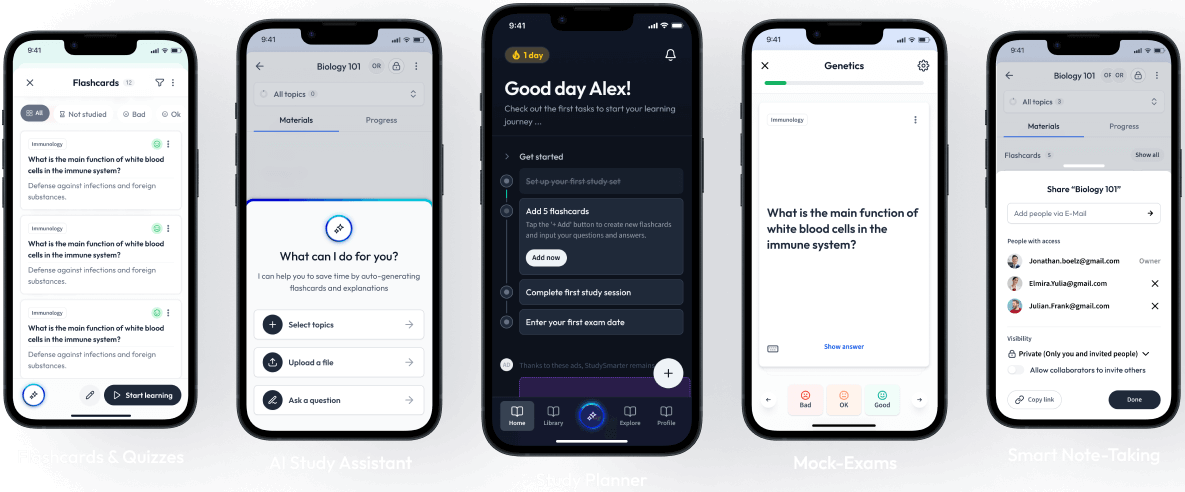
Sign up to highlight and take notes. It’s 100% free.
Save explanations to your personalised space and access them anytime, anywhere!
Sign up with Email Sign up with AppleBy signing up, you agree to the Terms and Conditions and the Privacy Policy of StudySmarter.
Already have an account? Log in