
StudySmarter - The all-in-one study app.
4.8 • +11k Ratings
More than 3 Million Downloads
Free
Americas
Europe
During the second world war, the American and British secret agencies came up with a so-called "L-pill," which could be given to operatives working beyond the front lines. The pill was usually built into a false tooth and contained potassium cyanide. If you bit the false tooth hard enough, the poisonous compound was released, allowing the agents to suicide themselves before they got captured and possibly tortured. Here is the structure of potassium cyanide. What can you tell me about its structure?



Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persönlichen Lernstatistiken
Jetzt kostenlos anmeldenDuring the second world war, the American and British secret agencies came up with a so-called "L-pill," which could be given to operatives working beyond the front lines. The pill was usually built into a false tooth and contained potassium cyanide. If you bit the false tooth hard enough, the poisonous compound was released, allowing the agents to suicide themselves before they got captured and possibly tortured. Here is the structure of potassium cyanide. What can you tell me about its structure?
 Fig. 1: Structure of KCN, Isadora Santos, StudySmarter Originals.
Fig. 1: Structure of KCN, Isadora Santos, StudySmarter Originals.
We can tell by the structure that C and N are bonded together, forming the cyanide ion (a nonmetallic anion). The potassium (K) atom is bonded to the cyanide ion. Potassium cyanide (KCN) is an interesting compound with ionic and covalent bonds! Compounds can be ionic or molecular compounds. What does this mean, and what type of compound is potassium cyanide? Keep reading to find out!
Let us dive into the properties of ionic and molecular compounds. You will also learn how these compounds are named and what makes them different from each other!
When a bond forms between a cation and an anion, we call it an ionic bond. Ionic bonds occur when the cation donates electrons to the anion so that way they can both have an entire outer shell.
An ionic bond is an electrostatic attraction between two oppositely charged ions formed when one atom transfers electrons to another.
For example, when sodium (Na) bonds with chlorine (Cl) to make the compound NaCl, the sodium ion (Na+) donates one electron to the chlorine ion (Cl-). Sodium has one valence electron, while chlorine has seven valence electrons. They both want to have an entire outer shell and become more stable. So, sodium gets rid of its single electron in the outer shell and gives it to chlorine since chlorine needs one electron to fill its outermost shell. Even atoms like to help others by giving away what they don't need to those who do!
 Fig. 2: The ionic bond between Sodium and Chlorine, Isadora Santos - StudySmarter
Fig. 2: The ionic bond between Sodium and Chlorine, Isadora Santos - StudySmarter
What keeps the ions in an ionic bond together? Electrostatic forces between the metal and the non-metal hold the atoms together in an ionic bond!
When a compound comprises a negative and a positive ion, they are considered an ionic compound. A positive ion is called a cation, whereas a negative ion is called an anion.
Metal ions lose electrons to form cations, while non-metals gain electrons to form anions.
Ionic compounds are composed of positive and negative ions.
Ionic compounds have the following properties:
They have strong electrostatic attractions.
They are hard and brittle.
Ionic compounds have a crystal lattice structure.
Ionic compounds have high melting and boiling points.
Ionic compounds can conduct electricity only when in liquids or if dissolved.
Electronegativity is an atom's ability to attract a shared pair of electrons. To determine whether a compound is ionic or not, we can take a look at the difference in electronegativity between the two atoms. We can use the periodic table to compare the electronegativity between two atoms, and if the difference between them is greater than 1.2, they will form an ionic compound! Notice that in the periodic table below, electronegativity increases across a period (from left to right) and decreases down a group.
Would AlH3 form an ionic compound?
First, look at the electronegativity values of Al and H: 1.61 and 2.20. The difference in electronegativity between these two atoms is 0.59, and therefore they would not form an ionic compound.
Would IF form an ionic compound?
The electronegativity value of I is 2.66, and F is 3.98. The difference in electronegativity between these two atoms is 1.32, so we can say that IF is an ionic compound.
When naming ionic compounds, there are specific rules that we need to follow:
We always write ionic compounds in the following form: cation + anion.
If the cation has more than one charge, we need to write the positive charge using roman numbers. We always need to state the oxidation number, except for groups 1, 2, and Al3+, Zn2+, Ag+, and Cd2+. For example, if we have Fe+3, then we would write its name as Iron (III), but if we have Zn2+, we would write its name as Zinc.
The anion will keep the beginning to its name, but -ide needs to be added to the end.
To make things easier, let's look at an example!
Name the following compound: Na2O
Since sodium is considered a cation and oxygen an anion, they will form an ionic compound! So, let's follow the rules above and name this compound!
Well, that was pretty easy! Unfortunately, not all compounds are that easy to name. When we come across polyatomic ions, the naming is slightly different. Most common polyatomic ions are negatively charged (anions), except for the ammonium ion (NH4+) and the mercury (I) ions (Hg2+2). When polyatomic ions are present, they will always keep their name! So, the easiest way to name compounds involving polyatomic ions is to memorize their names!
Polyatomic ions are formed when two or more atoms join together.
Here is a list of the most common polyatomic ions you might encounter:
Let's look at some problems involving polyatomic ions.
1) Name the following ionic compound: CoCO3
First, notice that CO3 is a polyatomic anion: CO3-2. Cobalt (Co) is a transition metal, so it can have many charges. Since there is a -2 charge on CO3-2, we can assume that the charge in Co is +2. In other words, Co+2 will give away two valence electrons, and CO3-2 will accept two valence electrons.
Since a polyatomic anion is present, we have to maintain its name. By looking at the list of polyatomic ions, we know that the name for CO3-2 is carbonate. So, the name of this compound will be Co+2 metal + polyatomic anion: Cobalt (II) carbonate.
2) Write the formula for the following ionic compound: Magnesium sulfate
We know that magnesium (Mg) cation has a charge of +2 and that sulfate is a type of polyatomic anion with the formula SO42- . Since the charge of both the cation and the anion is the same, they cancel each other, so we don't need to write it. So, the formula for magnesium sulfate would be MgSO4.
Now, let's look at the molecular compound nomenclature. Naming molecular compounds is easier than ionic compounds' nomenclature when it comes to naming them.
First, look at the first nonmetal and write its numerical prefix. However, if the first nonmetal has a prefix of 1, do not add the "mono" prefix.
Write the name of the first nonmetal.
Write the numerical prefix of the second nonmetal.
Write the base name of the second nonmetal and change the end to -ide.
The numerical prefixes that you need to learn if you haven't yet are the following:
Feeling confused? Let's look at some examples!
1) Name the following molecular compound: N2O4
The numerical prefix for nitrogen (N) is 2, and the numeral prefix for oxygen (O) is 4. The name of this compound would be dinitrogen tetroxide.
2) What would be the formula for Dibromine heptoxide?
By looking at the name, notice that bromine has the prefix "di," and oxide (oxygen) has the prefix "hepta." So, the correct formula for disulfur monochloride is Br2O7.
Now that we learned about the structure and properties of ionic compounds, let's look at what molecular compounds to learn how they differ from ionic compounds. When nonmetals are joined together by covalent bonds, they form molecular compounds. Instead of a cation giving away its electrons to an anion as it happens in ionic bonding, covalent bonding consists of sharing valence electrons between two atoms.
Molecular compounds are compounds held together by covalent bonds.
Covalent bonds are bonds that are formed by a shared pair of electrons.
To better understand how nonmetals form covalent bonds, let's look at the figure below. Here, one carbon atom bonds to two oxygen atoms to form carbon dioxide CO2. Carbon has four valence electrons, and oxygen has six valence electrons.
They both want to have full outer shells (8 electrons), so they share electrons between them! Each oxygen atom will share two electrons with carbon, and carbon will share two electrons with each oxygen atom.
Decide whether the following compounds are ionic or molecular:
To solve this question, you need to know what makes a compound ionic or molecular. We said before that ionic compounds consist of a cation and an anion, whereas molecular compounds possess covalent bonds.
Cu(NO3)2 is an ionic compound because Cu2+ is a cation, and NO3- is a polyatomic anion known as carbonate.
CCl4 is a molecular compound because both C and Cl are non-metals that are held together by covalent bonds.
Although (NH4)2SO4 looks like a molecular compound, remember that the ammonium ion (NH4+) is considered a polyatomic cation, and SO42- is a polyatomic anion. Since we have a cation and an anion, we can say that (NH4)2SO4 is an ionic compound.
Simple covalent molecules have low melting and boiling points. They are also insoluble in water and are considered poor conductors of electricity since they cannot carry a charge (they are neutral). Common examples of simple covalent molecules include CO2, O2, and NH4.
Simple covalent molecules are made up of small atoms covalently bonded.
Macromolecules are also called giant covalent structures. These compounds are also molecular compounds, but they have different properties. Macromolecules have high melting and boiling points, and they are hard and strong. They are also insoluble in water and are unable to conduct electricity. Some examples of macromolecules include silicon and diamond.
Macromolecules are lattices of atoms joined together by multiple covalent bonds in all directions. A lattice is a structure made of a repeating arrangement of particles.
So, why does cyanide kill you?
Cyanide poisoning occurs when a person gets exposed to high amounts of cyanide, which happens because cyanide gets absorbed into the body and binds the heme iron in cytochrome A3, blocking the mitochondrial electron transport. This then causes cellular hypoxia, which is referred to as the presence of lower oxygen content in the cell. Then, a metabolic switch to an anaerobic pathway occurs, causing lactic acidosis. Cyanide poisoning causes a person to suffocate and can lead to cardiac failure.
Let's talk a bit more about the conductivity of molecular and ionic compounds. Ionic compounds are capable of electrical conductivity only when molten or dissolved. When the ionic solid gets dissolved in water or when in its molten state, the ions separate and become free to move around and conduct electricity.
Covalent compounds, on the other hand, are incapable of conducting electricity because they have no charged particles that can freely move. The only exception is graphite. Graphite has loosely held electrons that can move through the solid structure, conducting electricity.
Now, let's take a look at examples involving ionic and molecular compounds. Some examples of ionic compounds include CuCl, and CuSO4.
Cuprous chloride (CuCl) is an ionic solid that has a melting point of 430 °C. In organic chemistry, CuCl can be used in a reaction with aromatic diazonium salts to form aryl chlorides. It can also be used as a catalyst in other organic reactions. Copper (II) sulfate is also an ionic solid, and it has a melting point of 200 °C. CuSO4 has many uses, such as a soil additive in agriculture and as a wood preservative.
Examples of molecular compounds include N2O4, and CO. Dinitrogen tetroxide (N2O4) is a gas at STP. It was a boiling point of 21.2 °C. N2O4 can be used as a fuel additive, for example, as a rocket propellant! Carbon monoxide (CO) is also a gas at STP, and it has a boiling point of -191.5 °C. Carbon monoxide can be very dangerous. For example, when a person gets CO poisoning, these carbon monoxide molecules bind to hemoglobin instead of oxygen molecules.
I hope you are more comfortable with ionic and molecular compounds now; maybe you can tell them apart by their specific properties!
A formula representing an ionic compound would be KCN, while a formula representing a molecular compound would be N2O4.
The difference between ionic and molecular compounds is that ionic compounds are composed of positive and negative ions held together by ionic bonds. In contrast, molecular compounds are compounds made up of nonmetals covalently bonded to each other.
To name ionic compounds, there are some rules you need to follow:
To name molecular compounds, the rules are:
Ionic compounds are composed of positive and negative ions held together by ionic bonds.
Molecular compounds are compounds made up of nonmetals covalently bonded to each other.
Ionic compounds are composed of positive and negative ions held together by ionic bonds. Examples of ionic compounds include KCN, NaCl, and Na2O.
Molecular compounds are compounds made up of nonmetals covalently bonded to each other. Examples of Molecular compounds include CCl4, CO2, and N2O5.
Flashcards in Ionic and Molecular Compounds419
Start learningDescribe metallic bonding.
The attraction between an array of positive metal ions and a sea of delocalized electrons.
What sort of elements bond using metallic bonding?
Just metals
True or false? Metals bond using shared pairs of electrons.
False
Explain why magnesium experiences stronger metallic bonding than sodium.
Explain why metals are good conductors of electricity.
They contain delocalized electrons which are free to move and carry a charge.
Explain why metals are lustrous.
Metals contain delocalized electrons. These absorb light energy and become excited. To return to their ground state, they release the energy as light, giving off a lustrous gleam.
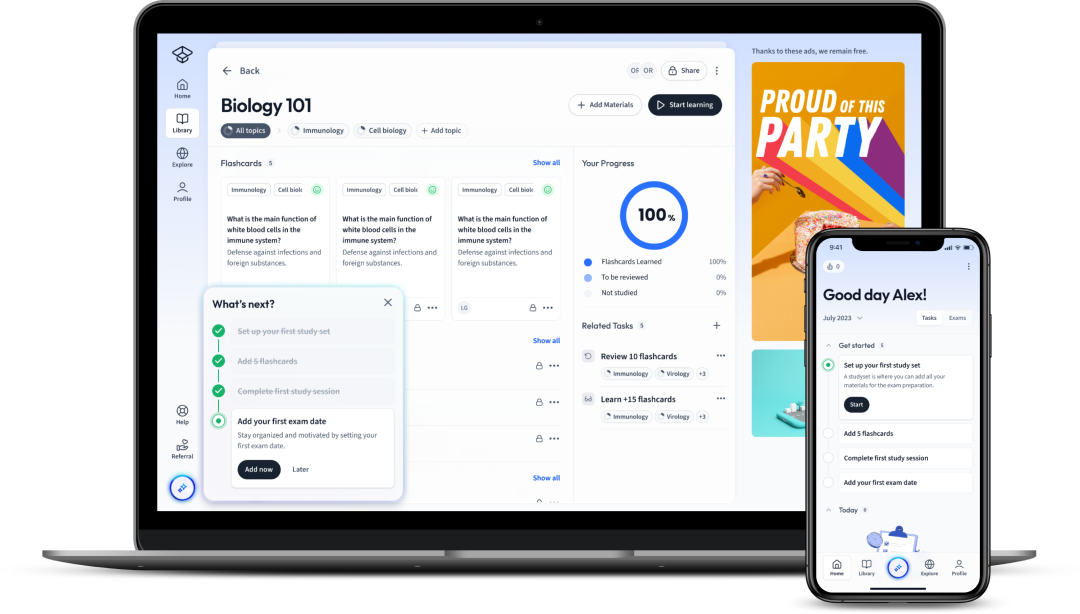
Already have an account? Log in
Open in AppThe first learning app that truly has everything you need to ace your exams in one place
Sign up to highlight and take notes. It’s 100% free.
Save explanations to your personalised space and access them anytime, anywhere!
Sign up with Email Sign up with AppleBy signing up, you agree to the Terms and Conditions and the Privacy Policy of StudySmarter.
Already have an account? Log in